Kaiser Health News
Georgia’s Medicaid Work Requirements Costing Taxpayers Millions Despite Low Enrollment
Andy Miller and Renuka Rayasam
Wed, 20 Mar 2024 09:00:00 +0000
Georgia Gov. Brian Kemp's plan for a conservative alternative to Obamacare's Medicaid expansion has cost taxpayers at least $26 million so far, with more than 90% going toward administrative and consulting costs rather than medical care for low-income people.
Kemp's Georgia Pathways to Coverage offers government health insurance to people earning up to the federal poverty level — $15,060 for an individual adult — if they can document that they're working, in school, or performing other qualifying activities.
Since July, when the program began, about 3,500 people have signed up, according to state officials. That's a small fraction of the Georgians who could enroll if the state expanded Medicaid without such requirements.
Republican leaders in several states have sought to require that people who are eligible for Medicaid through expansion work, arguing the health program for low-income Americans shouldn't be a handout. Kemp's experiment, aimed at single adults with low incomes who aren't already eligible for Medicaid, is the only current effort to survive legal challenges. But critics say it creates obstacles for people in need of health care while wasting taxpayer dollars on technology, consultants, and attorney's fees.
The Pathways program is “fiscally foolish and anti-family,” said Joan Alker, executive director and co-founder of Georgetown University's Center for Children and Families. She noted that full-time caregiving does not qualify someone for eligibility into the program. “A lot of taxpayer money has been wasted,” she said, “and not on health care for people who need it.”
The state projected that administrative costs will increase to $122 million over four years, mostly in federal spending, as it rolls out key features of the program, including the collection of premiums and verifying enrollees' eligibility, according to an internal planning document dated December 2022 obtained by KFF Health News. The primary consultant for the project is Deloitte, which is collecting hefty fees.
Georgia's GOP-led state legislature has rejected what Democrats say would be a far simpler way to cover the state's low-income workers: expanding Medicaid under the Affordable Care Act. That could make at least 359,000 uninsured people in Georgia newly eligible for Medicaid, according to KFF data. In addition, Georgia could reduce state spending by $710 million over two years, according to KFF research from 2021.
Despite Georgia's rocky implementation experience, state Republican leaders have put off considering a full Medicaid expansion. And such conservative states as Mississippi, Idaho, and South Dakota are weighing similar work requirements.
“You're spending money, primarily here, to put people through an extra set of hoops before they get coverage,” said Benjamin Sommers, a professor of health care economics at Harvard T.H. Chan School of Public Health.
The low enrollment for Pathways has disappointed supporters, as the state projected more than 25,000 residents would enroll during its first year and 52,000 by the end of five years, according to its application to the federal government.
Chris Denson, director of policy and research at the conservative Georgia Public Policy Foundation, which supports Pathways, said the low enrollment numbers are “just part of the ramping up.”
The program was intended to start in July 2021 but was delayed two years due to legal wrangling. In December 2022, Georgia officials told the federal Centers for Medicare & Medicaid Services that it would cost at least $51 million over two years to design, develop, and implement an eligibility system, funds that would largely be channeled to Deloitte Consulting, according to the documents KFF Health News obtained.
About 45% of Pathways applications were still waiting to be processed, based on the state's most recent monthly reports, said Leah Chan, director of health justice at the Georgia Budget and Policy Institute, a nonprofit research organization that supports full Medicaid expansion.
The eligibility system, she said, “the thing that we've spent the most money on, is actually one of the things standing in the way of the program seeing higher enrollment.”
The state Department of Community Health reported $26.6 million in Pathways spending through Dec. 31, of which more than 80% was paid for using federal funds. Deloitte was paid $2.4 million to prepare and submit the application to the federal government. Just $2 million was paid to insurers to cover medical care. In the fourth quarter, administrative costs alone rose by more than $6 million.
The total costs do not include legal fees for defending the Pathways program. The state attorney general's office said that as of Feb. 7 those costs surpass $230,000.
In striking contrast, North Carolina has enrolled 380,000 beneficiaries in its Medicaid expansion as of March 1, according to that state's Department of Health and Human Services. North Carolina became the 40th state to expand Medicaid under the ACA on Dec. 1, a move that has prompted fresh debate over expansion in a handful of other Southern holdout states.
Georgia, which has one of the highest uninsured rates among states, is currently the only state that requires people in its Medicaid expansion population to prove they are working or doing other qualifying activities to gain health coverage.
A spokesperson for Kemp, Carter Chapman, told KFF Health News that the governor “remains committed to implementing Georgia Pathways, an innovative program expanding coverage to tens of thousands of otherwise ineligible, low-income Georgians, despite the Biden administration's continued efforts to disrupt its rollout.”
In February, citing the delays in implementation, Georgia filed a suit against the federal government to ensure the work requirement program could continue running through 2028 instead of 2025, when it was originally scheduled to end. CMS refused to comment because of pending litigation.
Georgia's cost estimates are in line with what other states anticipated for administrative spending for Medicaid work requirement programs, including Kentucky's projected spending of $272 million, according to a 2019 report from the Government Accountability Office, a federal agency that recommended CMS consider administrative costs in such applications.
In Arkansas, administrative costs for the state's work requirement program were nearly 30% higher than costs of running standard Medicaid in 2016, according to a report from the Arkansas Center for Health Improvement, a nonpartisan health policy group in the state. People struggled to prove they qualified because setting up online accounts was difficult and confusing and many had limited access to the internet, said Robin Rudowitz, a vice president at KFF and director of the Program on Medicaid and the Uninsured. Arkansas' work requirement program ended in 2019 after a judge blocked it, but not before 18,000 people lost coverage. Unlike Arkansas, which placed a work requirement on a population already receiving Medicaid expansion benefits, Georgia is offering coverage to new people who qualify. But the program's expense may not be worth sustaining it, Sommers said.
Typically, in Medicaid, administrative costs range from 12% to 16% of overall program spending, said Laura Colbert, executive director of the advocacy group Georgians for a Healthy Future, which supports full Medicaid expansion.
“It's reasonable to expect that at least 80% of costs of a public or private health insurance plan to go toward health care and services,” she said.
——————————
By: Andy Miller and Renuka Rayasam
Title: Georgia's Medicaid Work Requirements Costing Taxpayers Millions Despite Low Enrollment
Sourced From: kffhealthnews.org/news/article/georgia-medicaid-work-requirements-experiment-high-cost-low-enrollment/
Published Date: Wed, 20 Mar 2024 09:00:00 +0000
Did you miss our previous article…
https://www.biloxinewsevents.com/needle-pain-is-a-big-problem-for-kids-one-california-doctor-has-a-plan/
Kaiser Health News
First Responders, Veterans Hail Benefits of Psychedelic Drugs as California Debates Legalization
Bernard J. Wolfson
Mon, 13 May 2024 09:00:00 +0000
Wade Trammell recalls the time he and his fellow firefighters responded to a highway crash in which a beer truck rammed into a pole, propelling the truck's engine through the cab and into the driver's abdomen.
“The guy was up there screaming and squirming. Then the cab caught on fire,” Trammell says. “I couldn't move him. He burned to death right there in my arms.”
Memories of that gruesome death and other traumatic incidents he had witnessed as a firefighter in Mountain View, California, didn't seem to bother Trammell for the first seven years after he retired in 2015. But then he started crying a lot, drinking heavily, and losing sleep. At first, he didn't understand why, but he would later come to suspect he was suffering from post-traumatic stress disorder.
After therapy failed to improve his mental well-being, he heard about the potential benefits of psychedelic drugs to help first responders with PTSD.
Last July, Trammell went on a retreat in Puerto Vallarta, Mexico, organized by The S.I.R.E.N. Project, a nonprofit that advocates the use of psychedelics and other alternative medicines to help first responders. He took psilocybin mushrooms and, the next day, another psychedelic derived from the toxic secretions of the Sonoran Desert toad. The experience, he says, produced an existential shift in the way he thinks of the terrible things he saw as a firefighter.
“All that trauma and all that crap I saw and dealt with, it's all very temporary and everything goes back into the universe as energy,” Trammell says.
Abundant research has shown that psychedelics have the potential to produce lasting relief from depression, anxiety, PTSD, addiction, and other mental health conditions. Many universities around the United States have programs researching psychedelics. But experts warn that these powerful drugs are not for everybody, especially those with a history of psychosis or cardiovascular problems.
Most psychedelic drugs are prohibited under federal law, but California may soon join a growing number of local and state governments allowing their use.
A bill working its way through the California Legislature, would allow the therapeutic use of psilocybin; mescaline; MDMA, the active ingredient in ecstasy; and dimethyltryptamine, the active ingredient in ayahuasca, a plant-based psychoactive tea. The drugs could be purchased and ingested in approved locations under the supervision of facilitators, who would undergo training and be licensed by a new state board. The facilitators would need a professional health credential to qualify.
The bill, co-sponsored by Sen. Scott Wiener (D-San Francisco), Assembly member Marie Waldron (R-San Diego), and several other lawmakers, follows last year's unsuccessful effort to decriminalize certain psychedelics for personal use. Gov. Gavin Newsom, a Democrat, vetoed that bill, though he extolled psychedelics as “an exciting frontier” and asked for new legislation with “regulated treatment guidelines.”
Wiener says the new bill was drafted with Newsom's request in mind. It is supported by some veterans and first responder groups and opposed by numerous law enforcement agencies.
One potential roadblock is the state's budget deficit, pegged at between $38 billion and $73 billion. Newsom and legislative leaders may choose not to launch a new initiative when they are cutting existing programs. “That is something we'll certainly grapple with,” Wiener says.
The legislation, which is making its way through committees, would require the new board to begin accepting facilitator license applications in April 2026. The system would look somewhat like the one in Oregon, which allows the use of psilocybin mushrooms under the guidance of state-licensed facilitators at psilocybin service centers. And like Oregon, California would not allow for the personal use or possession of psychedelics; the drugs would have to be purchased and consumed at the authorized locations.
Colorado, following the passage of a ballot initiative in 2022, is creating a system of regulated “healing centers,” where people will be able to legally consume psilocybin mushrooms and some other psychedelics under the supervision of licensed facilitators. Colorado's law allows for the personal use and possession of a handful of psychedelics.
In California, the cities of Oakland, San Francisco, Berkeley, Santa Cruz, and Arcata have effectively decriminalized many psychedelics, as have other cities around the United States, including Ann Arbor, Michigan; Cambridge, Massachusetts; Detroit; Minneapolis; Seattle; and Washington, D.C.
Psychedelics such as psilocybin, ayahuasca, and peyote have been used for thousands of years by Indigenous populations in Latin America and the current-day United States. And some non-Indigenous groups use these substances in a spiritual way.
The Church of Ambrosia, with locations in San Francisco and Oakland, considers psilocybin mushrooms, also known as magic mushrooms, a sacrament. “Mushrooms affect the border between this world and the next, and allow people to connect to their soul,” says Dave Hodges, founder and pastor of the church.
Hodges was behind an unsuccessful attempt to get an initiative on the California ballot this year that would have decriminalized the possession and use of mushrooms. He hopes it will qualify for the 2026 ballot.
The pending California legislation is rooted in studies showing psychedelics can be powerful agents in mental health treatment.
Charles Grob, a psychiatry professor at the University of California-Los Angeles School of Medicine who has researched psychedelics for nearly 40 years, led a study that found synthetic psilocybin could help reduce end-of-life anxiety in patients with advanced-stage cancer.
Grob says MDMA is good for couples counseling because it facilitates communication and puts people in touch with their feelings. And he conducted research in Brazil that showed ayahuasca used in a religious context helped people overcome alcoholism.
But Grob warns that the unsupervised use of psychedelics can be dangerous and says people should undergo mental and medical health screenings before ingesting them. “There are cases of people going off the rails. It's a small minority, but it can happen, and when it does happen it can be very frightening,” Grob says.
Ken Finn, past president of the American Board of Pain Medicine, says that psychedelics have a number of side effects, including elevated blood pressure, high heart rate, and vomiting, and that they can trigger “persistent psychosis” in a small minority of users. Legal drugs also pose risks, he says, “but we have much better guardrails on things like prescriptions and over-the-counter medications.” He also worries about product contamination and says manufacturers would need to be tightly regulated.
Another potential problem is health equity. Since insurance would not cover these sessions, at least initially, they would likely attract people with disposable income. A supervised psilocybin journey in Oregon, for example, can cost more than $2,500.
Many people who have experienced psychedelics corroborate the research results. Ben Kramer, a former Marine who served in Afghanistan and now works as a psilocybin facilitator in Beaverton, Oregon, says a high-dose mushroom session altered his worldview.
“I relived the first time I was ever shot at in Afghanistan,” he says. “I was there. I had this overwhelming love and compassion for the guy who was shooting at me, who was fighting for what he believed in, just like I was.”
Another characteristic of psychedelic therapy is that just a few sessions can potentially produce lasting results.
Trammell, the retired firefighter, hasn't taken psychedelics since that retreat in Mexico 10 months ago. “I just felt like I kind of got what I needed,” he says. “I've been fine ever since.”
This article was produced by KFF Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation.
——————————
By: Bernard J. Wolfson
Title: First Responders, Veterans Hail Benefits of Psychedelic Drugs as California Debates Legalization
Sourced From: kffhealthnews.org/news/article/first-responders-veterans-psychedelic-drugs-california-legalization/
Published Date: Mon, 13 May 2024 09:00:00 +0000
Did you miss our previous article…
https://www.biloxinewsevents.com/fda-said-it-never-inspected-dental-lab-that-made-controversial-agga-device/
Kaiser Health News
FDA Said It Never Inspected Dental Lab That Made Controversial AGGA Device
Brett Kelman and Anna Werner, CBS News
Mon, 13 May 2024 11:30:00 +0000
The FDA never inspected Johns Dental Laboratories during more than a decade in which it made the Anterior Growth Guidance Appliance, or “AGGA,” a dental device that has allegedly harmed patients and is now the subject of a criminal investigation.
According to FDA documents obtained through the Freedom of Information Act, the agency “became aware” of the AGGA from a joint investigation by KFF Health News and CBS News in March 2023, then responded with its first-ever inspection of Johns Dental months later.
That inspection found that the Indiana dental device manufacturer didn't require all customer complaints to be investigated and the company did not investigate some complaints about people being hurt by products, including the AGGA, the FDA documents state. The FDA requires device companies to investigate complaints and forward them to the agency. Johns Dental had “never” alerted the FDA to any such complaints, according to the documents.
The AGGA, which its inventor testified has been used on more than 10,000 patients, was promoted by dentists nationwide, some of whom said it could “grow” or “expand” an adult's jaw without surgery and treat common ailments like sleep apnea. But these claims were not backed by peer-reviewed research, and Johns Dental has settled lawsuits from 20 patients who alleged the AGGA caused them grievous harm. The company has not admitted liability.
Two former FDA officials said the AGGA was likely able to stay on the market — and off the FDA's radar — for so long because of the lack of inspections and investigations at Johns Dental. Madris Kinard, a former FDA manager who founded Device Events, which analyzes FDA data, said it defies belief that Johns Dental never received a complaint worthy of relaying to the FDA.
“That's a red flag for me. If I don't see a single report to the FDA, I typically think there is something going on,” Kinard said. “When they don't report, what you have is devices that stay on the market much longer than they should. And patients get harmed.”
Johns Dental Laboratories declined to comment when reached by phone and its lawyers did not respond to requests for an interview. The family-owned company, which has operated since 1939 in the western Indiana city of Terre Haute, sells dozens of products to dentists and makes hundreds of retainers and sleep apnea appliances each month, according to its website.
Twelve of Johns Dental's products are registered with the FDA as Class II medical devices, meaning they carry at least a moderate risk, and some have been featured on the company website for at least two decades, according to screen captures preserved by the Internet Archive.
The AGGA, which was invented by Tennessee dentist Steve Galella in the 1990s, was not registered with the FDA like Johns Dental's other devices. Company owner Jerry Neuenschwander has said in sworn court depositions that Johns Dental started making the AGGA in 2012 and became Galella's exclusive manufacturer in 2015 and that at one point the AGGA was responsible for about one-sixth of Johns Dental's total sales revenue.
In another deposition, Johns Dental CEO Lisa Bendixen said the company made about 3,000 to 4,000 AGGAs a year and paid Galella's company a “royalty” of $50 to $65 for every sale.
“We are not dentists. We do not know how these appliances work. All we do is manufacture to Dr. Galella's specifications,” she said, according to a deposition transcript.
The FDA's lack of knowledge about the AGGA likely contributed to its loose oversight of Johns Dental. When asked to explain the lack of inspection, the FDA said that, based on what it knew at the time, it was not required to inspect Johns Dental until 2018 when the company registered as a “contract manufacturer” of other medical devices. Prior to 2018, the FDA was only aware of Johns Dental operating as a “dental laboratory,” which normally do not manufacture their own products and only modify devices made by other companies to fit dentists' specifications. The FDA does not regularly inspect dental labs, although it can if it has concerns or gets complaints, the agency said.
Kinard said that based on her experience at the FDA she believes the agency prioritizes medical devices over dental devices, which may have contributed to the lack of inspections at Johns Dental.
“There hasn't been much attention to dental devices in the past,” Kinard said. “Hopefully that's going to change because of dental implant failures, as well as this device, which has quite obviously had serious issues.”
The AGGA resembles a retainer and uses springs to apply pressure to the front teeth and upper palate, according to a patent application. Last year, the KFF Health News-CBS News investigation revealed the AGGA was not backed by any peer-reviewed research and had never been submitted to the FDA for review. At the time, at least 20 patients had alleged in lawsuits that the AGGA had caused grievous harm to their teeth, gums, and bone — and some said they'd lost teeth. Multiple dental specialists said in interviews that they had examined AGGA patients whose teeth had been shoved out of position by the device, sometimes causing tens of thousands of dollars in damage.
“The entire concept of this device, of this treatment, makes zero sense,” said Kasey Li, a maxillofacial surgeon who published research on AGGA patients that appeared on a National Institutes of Health website. “It doesn't grow the jaw. It doesn't widen the jaw. It just pushes the teeth out of their original position.
Johns Dental and Galella have negotiated out-of-court settlements with the original 20 AGGA plaintiffs without publicly admitting fault. At least 13 more AGGA patients have filed similar lawsuits since the KFF Health News-CBS News investigation. Johns Dental and Galella denied wrongdoing or have not yet responded to the allegations in the newer lawsuits.
Galella declined to be interviewed in 2023 and neither he nor his attorneys responded to recent requests for comment. One of his attorneys, Alan Fumuso, said in a 2023 statement that the AGGA “is safe and can achieve beneficial results” when used properly.
In the wake of the KFF Health News-CBS News report, Johns Dental abruptly stopped making the AGGA, according to the newly released FDA documents. The Department of Justice soon after opened a criminal investigation into the AGGA that was ongoing as of December, according to court filings. No charges have been filed. A DOJ spokesperson declined comment.
Spurred by the March 2023 news report, the FDA inspected Johns Dental in July. The FDA's website shows that Johns Dental was issued seven citations, but the substance of the agency's findings was not known until the inspection report was obtained this year.
FDA investigator David Gasparovich wrote in that report that he arrived unannounced at Johns Dental last July and was met by five attorneys who instructed employees not to answer any questions about the AGGA or the company's complaint policies. Neuenschwander was told by his attorney not to talk to the inspector, the report states.
“He asked if he could photograph my credentials,” Gasparovich wrote in his report. “This was the last conversation I would have with Mr. Neuenschwander at the request of his attorney.”
The FDA requires device companies to investigate product complaints and submit a “medical device report” to the agency within 30 days if the products may have contributed to serious injury or death. Gasparovich's inspection report states that Johns Dental had “not adequately investigated customer complaints,” and its complaint policies were “not adequately established,” allowing employees to not investigate if the product was not first returned to the company.
Johns Dental received four complaints about the AGGA after the KFF Health News-CBS News report, including one that came after the FDA announced “safety concerns” about the device, according to the inspection report.
“Zero (0) out of the four (4) complaints were investigated,” Gasparovich wrote in the report. “Each complaint was closed on the same day it was received.”
In the months after Gasparovich's inspection, Johns Dental sent letters to the FDA saying it revised its complaint policies to require more investigations and hired a consultant and an auditor to address other FDA concerns, according to the documents obtained through FOIA.
Former FDA analyst M. Jason Brooke, now an attorney who advises medical device companies, said the FDA uses an internal risk-based algorithm to determine when to inspect manufacturers and he advises his clients to expect inspections every three to five years.
Brooke said the AGGA is an example of how the FDA's oversight can be hamstrung by its reliance on device manufacturers to be transparent. If device companies don't report to the agency, it can be left unaware of patient complaints, malfunctions, or even entire products, he said.
When a company “doesn't follow the law,” Brooke said, “the FDA is in the dark.”
“If there aren't complaints coming from patients, doctors, competitors, or the company itself, then in a lot of ways, there's just a dearth of information for the FDA to consume to trigger an inspection,” Brooke said.
CBS News producer Nicole Keller contributed to this article.
——————————
By: Brett Kelman and Anna Werner, CBS News
Title: FDA Said It Never Inspected Dental Lab That Made Controversial AGGA Device
Sourced From: kffhealthnews.org/news/article/fda-inspection-johns-dental-agga-device/
Published Date: Mon, 13 May 2024 11:30:00 +0000
Did you miss our previous article…
https://www.biloxinewsevents.com/san-francisco-tries-tough-love-by-tying-welfare-to-drug-rehab/
Kaiser Health News
San Francisco Tries Tough Love by Tying Welfare to Drug Rehab
Ronnie Cohen
Mon, 13 May 2024 09:00:00 +0000
Raymond Llano carries a plastic bag with everything he owns in one hand, a cup of coffee in the other, and the flattened cardboard box he uses as a bed under his arm as he waits in line for lunch at Glide Memorial Church in San Francisco. At 55, he hasn't had a home for 15 years, since he lost a job at Target.
Llano once tried to get on public assistance but couldn't — something, he said, looking perplexed, about owing the state money — and he'd like to apply again.
But beginning next year, if he does, he'll face a new city requirement that single adults with no dependents who receive cash benefits be screened for illegal drug use and, if deemed necessary, enter treatment. San Francisco's voters approved the new mandate in March.
Llano has no objection to being screened. He said he uses cannabis, which is legal in California, though not federally, but does not use other drugs. Nonetheless, he said, “I suppose I would try recovery.”
Another man in the free-lunch line, Francis Farrell, 56, was far less agreeable. “You can screen me,” he said, raising his voice, “but I don't think you should force me into your idea of treatment.”
No one will be forced to undergo substance abuse treatment, nor will anyone be subject to drug testing, San Francisco officials insist. Rather, starting in January 2025, San Francisco's public assistance recipients who screen positive for addiction on a 10-question drug abuse test will be referred to treatment. Those who refuse or fail to show up for treatment will forfeit the $109 a month that the city grants to homeless adults who qualify for city shelters or supportive housing, or the $712 a month it grants to adults with home addresses.
The city famous for its tolerance is resorting to tough love.
Trent Rhorer, executive director of the San Francisco Human Services Agency, cited three reasons for the new measure, which was fashioned after similar policies in Los Angeles and New York: to incentivize people with a substance use disorder to enter treatment, to prevent taxpayer money from being used to buy illegal drugs, and to dissuade drug seekers from moving to San Francisco.
“We're giving them the opportunity to engage in something, without requiring sobriety, to hopefully get on a path to recovery,” Rhorer told KFF Health News.
When Mayor London Breed introduced the ballot initiative known as Measure F in a news conference last year, she called it an incentive to encourage drug-addicted recipients of public assistance to enter “into a program that will help save their life.” Accidental overdoses killed more than 800 people in San Francisco last year.
But in the eyes of many health care providers, researchers, and harm reduction advocates, the measure is neither an incentive nor an opportunity.
The policy was designed to have “a coercive, punitive effect” and could do more harm than good, said Vitka Eisen, president and chief executive of HealthRIGHT 360, San Francisco's largest drug treatment provider.
“It would have been an interesting project, much more in the spirit of San Francisco as a hub of innovation, to figure out if we can identify people with substance use disorder. And if they go into treatment and stay for a period of time, they'll get an increased benefit,” Eisen said.
About 5,800 people in the city currently receive benefits from the County Adult Assistance Programs, or CAAP. Under Measure F, those who acknowledge drug abuse on the screening test but refuse treatment and live in city-provided shelter will lose their cash benefits but can maintain their shelter, Rhorer said. However, CAAP recipients who refuse treatment and depend on public assistance to pay their rent in private housing could lose their homes.
The city will give recipients three chances to show up for treatment and will pay rent directly to a landlord for one month, Rhorer said. Measure F came in response to the grim conditions on some San Francisco streets, where men and women lie on sidewalks, often blocking passersby with their arms and legs splayed, or stand bent over, frozen like statues. Many use fentanyl, a synthetic opioid that has turned a long-standing homelessness problem into a public health emergency.
About 12% of people who fatally overdosed in San Francisco last year were CAAP recipients, Rhorer said.
Compassion fatigue seems to have settled over this city known for its kindheartedness. Measure F proponents raised $667,000 — more than 17 times as much as opponents — largely from business executives and tech investors, according to the San Francisco Ethics Commission. Then in March, 58% of voters approved the measure.
Since fentanyl began replacing heroin around 2019, Rhorer said, “drug tourists” have flocked to San Francisco, where the opioid has been cheap and plentiful. Lenient law enforcement and relatively generous cash public assistance grants also have drawn people with addiction, he said, although police activity has increased since last spring.
A recent city report found that only 53% of the 718 people whom police cited for substance use over a 10-month period that ended in February said they lived in the city.
“People who live in San Francisco, who really need the most help, don't get the help they need due to the influx of people coming from somewhere else,” said Cedric Akbar, who runs recovery programs and co-founded Positive Directions Equals Changes. “And should our tax dollars go to the ones in San Francisco, or are we going to take care of the whole country?”
Akbar began using heroin when he moved to San Francisco from Houston in the 1980s and has been in recovery for 31 years. He said he would have preferred even stricter requirements for eligibility for public assistance than those in Measure F but hopes the new mandate will at least help give people access to treatment.
The city's capacity for treatment is also a concern. Eisen and others describe a dire shortage of behavioral health workers to staff treatment facilities and residential step-down units, which are crucial for housing those in recovery from drug addiction.
New programs funded by the recently approved Proposition 1 in California, which authorizes the state to spend $6.38 billion to build mental health treatment facilities and provide housing for homeless people, are meant to address the shortages.
Leslie Suen, an addiction medicine physician and an assistant professor at the University of California-San Francisco, fears that pushing CAAP recipients into treatment could turn them off. When people “were stigmatized, or coerced, or told they would face consequences if they didn't do a certain thing,” she said, “that pushed them away from the health system even further.”
Though evidence suggests compulsory treatment can provide short-term benefits, it also can lead to long-term harm, the National Institute on Drug Abuse said in an email.
“To achieve the best outcomes,” the email said, treatment should be “delivered without stigma or penalty.”
Almost everyone with a substance use disorder enters treatment under some kind of pressure, whether from a parent, a spouse, an employer, or the criminal justice system, said Keith Humphreys, a Stanford University psychiatry professor.
Nonetheless, he questioned the morality of requiring welfare recipients, as opposed to criminals, to get drug treatment.
“I would never start with people who are poor but not committing crimes,” he said. “I would start with people who are harming others.”
This article was produced by KFF Health News, which publishes California Healthline, an editorially independent service of the California Health Care Foundation.
——————————
By: Ronnie Cohen
Title: San Francisco Tries Tough Love by Tying Welfare to Drug Rehab
Sourced From: kffhealthnews.org/news/article/san-francisco-welfare-drug-rehab/
Published Date: Mon, 13 May 2024 09:00:00 +0000
Did you miss our previous article…
https://www.biloxinewsevents.com/democrats-seek-to-make-gop-pay-for-threats-to-reproductive-rights/
-
SuperTalk FM5 days ago
Mississippi governor approves bill allowing electronic search warrants
-
SuperTalk FM7 days ago
Legislation outlawing ‘squatted’ vehicles in Mississippi signed into law
-
228Sports6 days ago
PRC’s Bats Come Alive Late As Blue Devils Beat Picayune To Advance To 6A South State Title Series
-
Mississippi News5 days ago
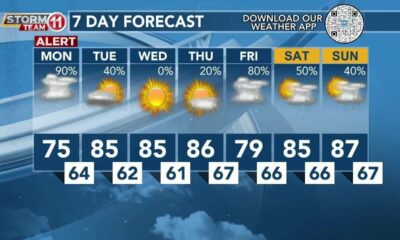
Strong storms late Wednesday night – Home – WCBI TV
-
Mississippi News5 days ago
Louisville names street after a former high school
-
SuperTalk FM5 days ago
$30 million RV park coming to Natchez features amphitheater, pickle ball courts, and more
-
Mississippi News5 days ago
Crews close Jackson street due to large sinkhole
-
Mississippi News4 days ago
Man arrested for allegedly breaking into home, robbing owner