Hailshadow via Gett Images
Daniel Pastula, University of Colorado Anschutz Medical Campus
The measles outbreak that started in Texas in late January continues to grow. As of March 18, 2025, confirmed cases in the outbreak, which now spans Texas, New Mexico and Oklahoma, reached 321, surpassing the number of confirmed cases recorded for all of the U.S. in 2024. The vast majority of cases are in people who are not vaccinated. Meanwhile, a lack of clarity from health authorities is leaving people with questions about whether they need to get revaccinated.
In a Q&A with The Conversation U.S., Daniel Pastula, a neurologist and medical epidemiologist from the University of Colorado Anschutz Medical Campus and Colorado School of Public Health, explained how and when you should take action.
Should adults get another shot of the measles vaccine?
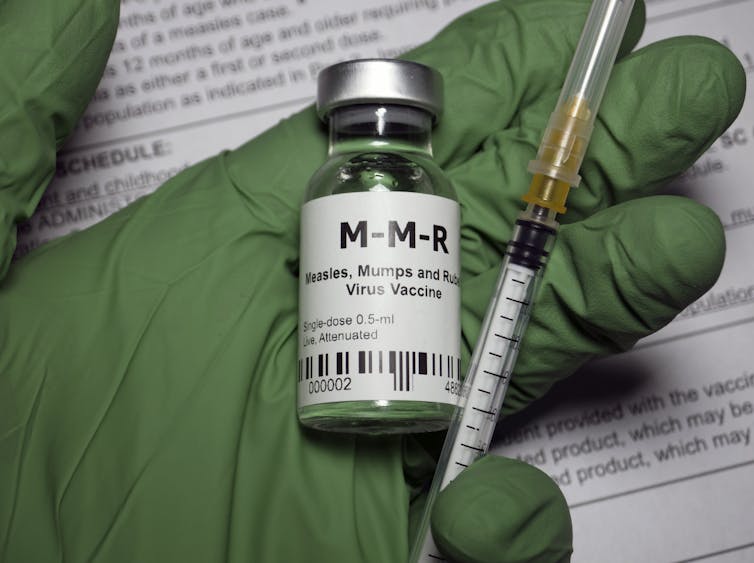
The measles vaccine, which first became available in the U.S. in 1963, contains a live but significantly weakened strain of the measles virus. This modified strain is too weak to cause measles, but it is similar enough to the wild type measles virus to train the immune system to recognize it. Most people who have received the live measles vaccine won’t need an additional shot now, but here is what you need to know:
People born before 1957 are presumed to have lifelong immunity because measles was so contagious that almost everyone contracted it before age 15. Unless there are special circumstances, they probably don’t need a vaccine now.
Most people born after 1957 would have received the shot as children, so they should be set for life. Physicians and public health experts don’t recommend most people in this group get a second measles shot, though there are exceptions.
In 1989, a limited outbreak of measles occurred among vaccinated school children. In response, the recommendations changed from one dose of the live measles vaccine to two doses for children. People fully vaccinated as children after that year do not need any additional doses.
Exceptions to these guidelines
There are two special circumstances where the previous recommendations may not hold.
First, if you were vaccinated between 1963 and 1967, one of the measles vaccines available at the time consisted of just proteins from the virus rather than a live, weakened version of it. Researchers soon realized this inactivated, or “killed,” vaccine was less effective and didn’t provide long-term immunity. Unless you know for certain you received the live vaccine, physicians and public health experts recommend that people vaccinated during those years get one dose of the live vaccine at some point.
Second, if you fall into a high-risk group – for example, if you are a health care provider, are traveling internationally or attending college, physicians and public health experts generally recommend getting a second dose if you have only had one.
For most adults without such risk factors, physicians and public health experts do not routinely recommend a second dose if you have previously received one dose of a live measles vaccine. If you have questions or concerns about your situation, make sure to ask your health care provider.
Except in very rare circumstances, there is no recommendation for a third dose of the measles vaccine.
Can you find out whether you’ve been vaccinated?
You might be able to! It’s worth checking. States actually keep vaccine records specifically for this reason, where you can look up your vaccine records or that of your kids. Your high school or college may still have your records, and so might your pediatrician’s office.
Should you get your antibody levels checked?
For most people, probably not.
A titer test checks the level of antibodies in your blood, and some people are asking their doctor to check their titers to determine whether they are still immune to measles. The problem is, the level of antibodies in your blood does not necessarily reflect your level of immunity. That’s because antibodies are just one part of your immune system’s infection-fighting force. Having a low level of antibodies does not necessarily mean your immunity has waned.
Other crucial elements of your immune response include B cells, T cells and other immune cells, but a titer test does not show their capabilities. For example, memory B cells might not currently be making antibodies against the virus but are primed to quickly do so the next time they see it. This is why antibody and titer tests should be used only in specific cases, in consultation with your doctor.
One example of when an antibody test may be warranted is if you are a health care provider born before 1957 and you want to make sure you don’t need another dose of the vaccine. You would use a test to see whether you have measles antibodies. But in this case you would be looking for a yes or no answer; the total amount of antibodies may not be very informative.
Is natural immunity better than vaccine-induced immunity?
Natural immunity – that is, the immunity you get after having measles – is effective. However, the downside is that natural infection with a wild virus is very risky. Before 1963, measles caused close to 50,000 hospitalizations and about 500 deaths each year in the United States, usually in children. It also caused over 1,000 cases of severe brain inflammation every year and carried several other long-term risks, such as permanent hearing loss or the wipe out of immunity to other diseases.
Bilanol via Getty Images
The point of vaccines is to create immunity without the risks of severe infection. It is basically a dress rehearsal for the real thing. The immunity from a vaccine is effectively the same immunity you get from having measles itself – but vastly safer than encountering the wild virus unprotected. One dose is 93% effective at preventing measles and two doses are 97% effective, and any breakthrough cases are likely to be much milder than a full-blown case of measles.
Can the vaccine cause measles?
No, the measles vaccine cannot cause measles because it contains a significantly weakened strain that has limited ability to infect and damage cells.
Some have claimed without evidence that the current outbreak in Texas was caused by the measles vaccine.
As part of the outbreak investigation, however, CDC and the Texas Department of State Health Services analyzed the genome of the virus causing the current outbreak and identified it as a wild measles virus. Researchers classify measles virus strains based on their genetic characteristics, or genotypes. They identified the outbreak virus as wild type genotype D8, and not the weakened measles vaccine strain, which is genotype A.
What are the risks of the vaccine?
That is a very reasonable question. Because the measles vaccine is a live, weakened virus strain, it can cause a mild, measles-like syndrome. For example, some people might have a slight fever, a rash, or some slight joint pain. These symptoms generally go away in a day or two, and most people don’t experience them. But the vaccine cannot cause measles itself, as it does not contain the wild measles virus.
In extremely rare cases, people can experience more significant reactions to the measles vaccine. It is important to remember that every single medical or health intervention carries risks – and that includes all medications and over-the-counter supplements. According to all available evidence, however, comparing the potential benefits against potential risks reveals that the risks of a signficant reaction to the vaccine are much lower than the risks of severe outcomes from measles itself.
Being vaccinated not only protects you and your family, but it also protects vulnerable people in the community, such as infants, cancer patients and pregnant women, who cannot be vaccinated themselves.
Daniel Pastula, Professor of Neurology, Medicine (Infectious Diseases), and Epidemiology, University of Colorado Anschutz Medical Campus
This article is republished from The Conversation under a Creative Commons license. Read the original article.