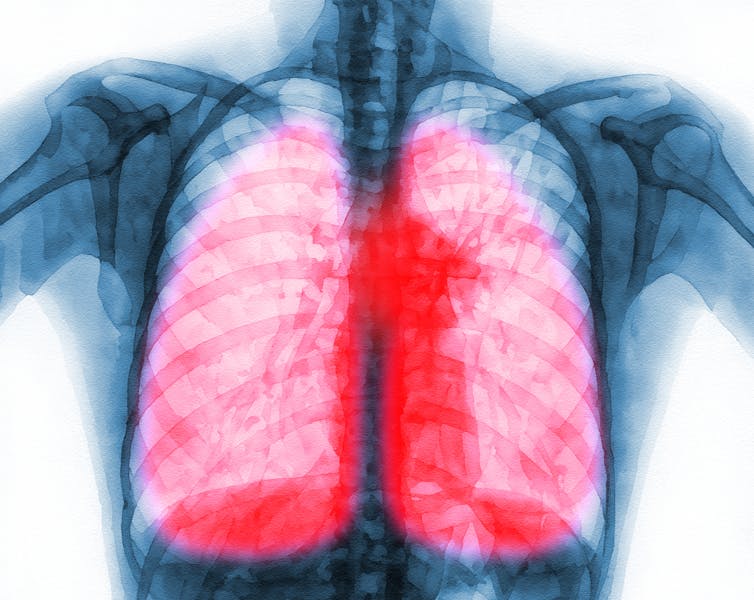
Many respiratory viral infections can cause long-term symptoms.
Harish Narasimhan, University of Virginia
The long-term effects of respiratory viral infections such as COVID-19 are a major public health burden. Some estimates suggest over 65 million people around the world suffer from long COVID-19.
Efforts to better understand this condition, however, have been hampered by its ability to affect multiple organ systems, such as those involving the lungs, brain and heart. This is further complicated by the lack of animal models that can sufficiently mimic the disease.
Animal models, such as mice and rats, are a crucial tool that researchers use to study human diseases and develop treatment strategies. Although there are major differences between humans and animal models, the vast majority of our immune and organs systems function similarly. Such similarities in physiology have made significant health care discoveries, including those related to COVID-19, possible.
I am an immunology researcher in the Sun Lab at the University of Virginia. We study the role the immune system plays in respiratory viral infections such as influenza and COVID-19. In our newly published research, we developed a new mouse model to study long COVID-19 and found that blocking certain overactive immune cells can restore lung function.
New models, new targets
Out team wanted to better understand the long-term effects of COVID-19 on the respiratory system. To do this, we worked to identify key features associated with lung scarring following COVID-19.
First, we examined lung samples from patients with long COVID-19. Although these patients were infected several months to years before the samples were taken, we found evidence of an overactive immune system in their lungs, particularly within areas that failed to fully repair themselves after infection.
Next, we aimed to create a mouse model for long COVID-19 by comparing the pathology of mice infected with four different types of respiratory viral infections. Surprisingly, we found that mice infected with influenza virus, rather than the COVID-19 mouse models scientists currently use, best replicated the physical features of severe chronic lung disease. The reasons why infections from different respiratory viruses affect the lungs in different ways are unclear. But preliminary evidence suggests it may be because each virus targets different types of cells “in humans and mice.”
Additionally, since long COVID-19 is about the damage left behind after infection, it seems less important what virus causes the problem in our animal model than that the damage is similar to what we want to address in human patients.
Using our new mouse model, we were able to identify the presence of an abnormal cluster of cells in mice lungs – made up of the same dysfunctional immune and epithelial, or structural, cells seen in the lungs of long-COVID-19 patients. Additionally, we found that the uncontrolled activity of these immune cells in the lungs impeded structural cells from repairing themselves. It also hindered them from restoring gas exchange, the process of taking in oxygen and releasing carbon dioxide.
Importantly, when we blocked the activity of proteins associated with this overactive immune response, it reduced lung scarring and restored optimal lung function in mice.
Treating respiratory viral infections
Most approaches to addressing long COVID-19 rely on starting treatment early after infection. To the best of our knowledge, our study is the first to identify strategies to treat the respiratory symptoms of long COVID-19 after this chronic disease develops.
The drugs we tested in our study have already been approved by the Food and Drug Administration to treat severe COVID-19 and other inflammatory conditions. We hope our findings can spur further research on using these drugs to treat long COVID-19.
Our work may have applications beyond long COVID-19. Growing evidence suggests that many respiratory viral infections, such as influenza, COVID-19 and respiratory syncytial virus, may result in chronic lung disease. Considering the four pandemics and even more respiratory viral epidemics that have occurred in the past 100 years, studying the cellular and molecular similarities between respiratory viral infections may be critical to how medical practitioners respond to future viral outbreaks.
Harish Narasimhan, Ph.D. Candidate in Immunology, University of Virginia
This article is republished from The Conversation under a Creative Commons license. Read the original article.